Targeting unmet needs in personalized oncology
XEPTAGEN SpA is engaged in the discovery and validation of novel molecular markers to improve the clinical management of cancer patients by creating innovative diagnostic tools that may allow a personalized treatment.
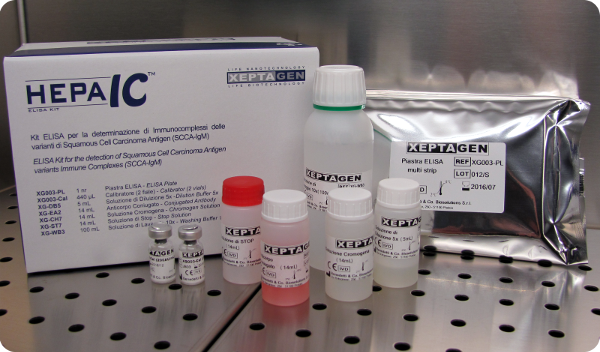
 Products Highlight
Products Highlight
 Latest Product Literature
Latest Product Literature
SCCA-IgM as a Potential Biomarker of Non-Alcoholic Fatty Liver Disease in Patients with Obesity, Prediabetes and Diabetes Undergoing Sleeve Gastrectomy. Obes Facts. 12:291306. 2019.
MiR-122 Targets SerpinB3 and Is Involved in Sorafenib Resistance in Hepatocellular Carcinoma.. J Clin Med. 8(2). 2019.
Squamous cell carcinoma antigen 1 is associated to poor prognosis in esophageal cancer through immune surveillance impairment and reduced chemosensitivity.. Cancer Sci.. 110(5):1552-1563. 2019.
Actual evidences and future perspectives about the role of iXip® in the diagnosis of prostate cancer: a narrative review.. Minerva Urol Nefrol. . 2019.
Clinical significances and diagnostic utilities of both miR-215 and squamous cell carcinoma antigen-IgM versus alpha-fetoprotein in Egyptian patients with hepatitis C virus-induced hepatocellular carcinoma.. Clin Exp Gastroenterol.. 12:51-66. 2019.
Diagnostic accuracy of SCCA and SCCA-IgM for hepatocellular carcinoma: A meta-analysis. Liver Int.. 38:18201831. 2018.
doi: 10.1159/000499717
MiR-122 Targets SerpinB3 and Is Involved in Sorafenib Resistance in Hepatocellular Carcinoma.. J Clin Med. 8(2). 2019.
doi: 10.3390/jcm8020171
Squamous cell carcinoma antigen 1 is associated to poor prognosis in esophageal cancer through immune surveillance impairment and reduced chemosensitivity.. Cancer Sci.. 110(5):1552-1563. 2019.
doi: 10.1111/cas.13986
Actual evidences and future perspectives about the role of iXip® in the diagnosis of prostate cancer: a narrative review.. Minerva Urol Nefrol. . 2019.
Clinical significances and diagnostic utilities of both miR-215 and squamous cell carcinoma antigen-IgM versus alpha-fetoprotein in Egyptian patients with hepatitis C virus-induced hepatocellular carcinoma.. Clin Exp Gastroenterol.. 12:51-66. 2019.
doi: 10.2147/CEG.S179832
Diagnostic accuracy of SCCA and SCCA-IgM for hepatocellular carcinoma: A meta-analysis. Liver Int.. 38:18201831. 2018.
doi: 10.1111/liv.13867
24.07.2018
iXip to reduce prostate rebiopsies Prostate biopsy is an invasive and expensive procedure, with a high risk of complications, especially infections.
A study published in Cancer Treatment and Research Communications (available Read more...
Prostate biopsy is an invasive and expensive procedure, with a high risk of complications, especially infections.
A study published in Cancer Treatment and Research Communications (available Read more...02.05.2018
A novel algorithm to detect NASH in HCV-positive patients A recent publication in the International Journal for Biological Markers describes the promising diagnostic performance of SCCA-IgM combined with common clinical data for the detection of non-alcoholi ... Read more...
A recent publication in the International Journal for Biological Markers describes the promising diagnostic performance of SCCA-IgM combined with common clinical data for the detection of non-alcoholi ... Read more...22.02.2018
Four communications on SCCA and SCCA-IgM at the 2018 AISF meeting The AISF annual meeting is taking place today and tomorrow in Rome.
The AISF annual meeting is taking place today and tomorrow in Rome.
This year, four presentations are dedicated to SCCA and SCCA-IgM: one oral communication is on the role of SCCA-IgM as a biomark ... Read more...31.01.2018
SCCA-IgM as a biomarker of liver disease in Asian patients A new study on SCCA-IgM in Asian patients has been published in the Scandinavian Journal of Clinical and Laboratory Investigation.
A new study on SCCA-IgM in Asian patients has been published in the Scandinavian Journal of Clinical and Laboratory Investigation.
SCCA-IgM was analyzed as a biomarker to monitor cirrh ... Read more...29.01.2018
New clinical study on iXip The new clinical study on iXip "PSAIgM2017" has been approved at the Spedali Civili hospital in Brescia, Italy.
The new clinical study on iXip "PSAIgM2017" has been approved at the Spedali Civili hospital in Brescia, Italy.
The prospective and multicenter study will analyze the correlation betwe ... Read more...25.10.2017
SCCA and SCCA-IgM for the diagnosis of perihilar cholangiocarcinoma During the Liver Meeting 2017 that ended yesterday in Washington DC, Gringeri et al. presented a study on SCCA and SCCA-IgM as biomarkers of perihilar cholangiocarcinoma (pCCA).
During the Liver Meeting 2017 that ended yesterday in Washington DC, Gringeri et al. presented a study on SCCA and SCCA-IgM as biomarkers of perihilar cholangiocarcinoma (pCCA).
The re ... Read more...19.06.2017
SCCA-IgM predicts the therapeutic response in patients with HCC Last week a prospective study demonstrating the ability of the biomarker SCCA-IgM to predict the response to therapy in patients with hepatocellular carcinoma (HCC) was published in the "Scandinavian ... Read more...
Last week a prospective study demonstrating the ability of the biomarker SCCA-IgM to predict the response to therapy in patients with hepatocellular carcinoma (HCC) was published in the "Scandinavian ... Read more...15.05.2017
SCCA-IgM for the monitoring of HCC in HCV-cirrhotic patients treated with direct-acting antivirals Hepatocellular carcinoma (HCC) risk persists after treatment with direct antiviral agents (DAAs) in patients with hepatitis C virus infection (HCV) and cirrhosis. Therefore, it is mandatory that patie ... Read more...
Hepatocellular carcinoma (HCC) risk persists after treatment with direct antiviral agents (DAAs) in patients with hepatitis C virus infection (HCV) and cirrhosis. Therefore, it is mandatory that patie ... Read more...02.05.2017
SCCA-IgM as a biomarker for Barrett esophagus and esophageal cancer A prospective study published in the Journal of Clinical Gastroenterology described the possible use of SCCA-IgM in the identification of patients at risk for Barrett esophagus (BE) and esophag ... Read more...
A prospective study published in the Journal of Clinical Gastroenterology described the possible use of SCCA-IgM in the identification of patients at risk for Barrett esophagus (BE) and esophag ... Read more...01.02.2017
New paper on iXip published A collaborative study published in the journal Cancer Treatment and Research Communications evaluated the diagnostic performance of iXip, XEPTAGEN's novel index to detect prostate cancer, in a ... Read more...
A collaborative study published in the journal Cancer Treatment and Research Communications evaluated the diagnostic performance of iXip, XEPTAGEN's novel index to detect prostate cancer, in a ... Read more...
Xeptagen SpA. - P.IVA 07784560638 - C.F. 03172070272 - Copyright © 2017. All rights reserved.
Sitemap. Disclaimer. Privacy Policy.